Ø Natural uranium is the raw material for producing enriched uranium and plutonium. Natural uranium fuel for graphite water cooled reactor and graphite gas cooled reactor is metal uranium added with a small amount of alloy elements. The natural uranium fuel for heavy water reactor is uranium dioxide ceramic body.
Ø With atomic number of 92 and atomic weight of 238, it is the heaviest element that can be found in nature.
Isotope Composition
Natural isotope composition of uranium is as follows:
Ø 238U: Natural abundance: 99.275%; atomic weight: 238.0508; half-life period: 4.51X109a
Ø 235U: Natural abundance: 0.720%; atomic weight: 235.0439; half-life period: 7.00X108a
Ø 234U: Natural abundance: 0.005%; atomic weight: 234.0409; half-life period: 2.47X105a
The abundance of U-235 required by the light-water reactor nuclear power station is about 3%~5%, and the abundance of U-235 required by nuclear weapons needs to be more than 90%. The abundance refers to the proportion of a certain isotope in this element. The abundance of U-235 is 3%, which shows that U-235 is having three of every 100 uranium nucleus. In accordance with different uranium concentrations, International Atomic Energy Agency (IAEA) divides uranium into micro-enriched uranium (0.9%~2%), low-enriched uranium (2%~20%) and high-enriched uranium (more than 20%). In the high-enriched uranium, U-235 with the abundance more than 85% is called weapon-grade enriched uranium which can be directly used for manufacturing nuclear weapons.
Uranium Element Distribution
With natural uranium extensively distributed in the crust, the average content of uranium in the crust is about 2.5ppm. In other words, the crust material per ton contains 2.5g of uranium averagely, which is higher than the content of tungsten, mercury, gold, silver and other elements. The contents of uranium are uneven in various kinds of rocks. For instance, its content in the granite is high, averagely 3.5g of uranium per ton. The content of uranium is almost 1.3×10^14t in the first layer of crust (20km from the earth surface). According to such estimate, one cubic kilometer of granite will contain about 10,000t of uranium. With low concentration in sea water, the sea water per ton averagely contains 3.3mg of uranium only. However, due to great total amount of sea water (the total uranium content may be 4.5×10^9t in the sea water) and its convenience to extract from the water, more than one country, especially those countries lack of uranium resources, is exploring in the method of extracting uranium from the sea water.
Ø There are three isotopes in nature, with half-life period lasting from hundreds of millions of years to billions of years. 238U amounts to 99.284% on earth.
Ø With active chemical property, it easily reacts with most non-metal elements.
Uranium Mineral
Ø Radioactive decay: The uranium nucleus is instable, and the chemical composition of uranium is inconstant.
Ø Isomorphism: On account that U4+ has a similar particle radius to that of Th4+ and REE3+, some U4+ minerals frequently contain mixed isomorphism matters of thorium and rare earth elements or exist in other mineral lattices in the form of isomorphism.
Ø Luminescence: Uranium mineral can be excited to emit visible light by the external energy (such as ultraviolet light). The hexavalent uranium, rather than the tetravalent uranium, has the luminescence.
Ø Valence state of uranium: It is present in the mineral in the valence states of +4 and +6. They have great differences both in crystal chemical properties and geochemical properties.
Ø Color: The tetravalent uranium mineral is mostly in red, brown-black or crineous. The hexavalent uranium mineral is mainly in yellow, yellow green and green.

Source: Chinese Nuclear Society.
There are more than 200 kinds of known uranium minerals, but only more than 10 uranium minerals with the industrial exploitation value, the most important of which is as follows:
Ø Uraninite
Ø Pitchblende
Ø Brannerite
Ø Autunite
Ø Carnotite
Ø Tyuyamunite
Genesis of Uranium Mineral
It mainly includes:
Ø Endogenic process (magmatism, germination, hydrothermalism, etc.)
Ø Hypergene process (epigenetic illuviation, weathering, etc.)
In general, the tetravalent uranium mineral is mainly an endogenous product, and the majority of hexavalent uranium is a hypergenic product.
Radioactivity of Uranium
Uranium is a radioactive nuclear material capable of emitting α, β and γ rays. But a small amount of radiation will not do harm to the human body. However, its radiation dose will be increased greatly to harm the human body when radioactive substances are enriched to a certain degree.
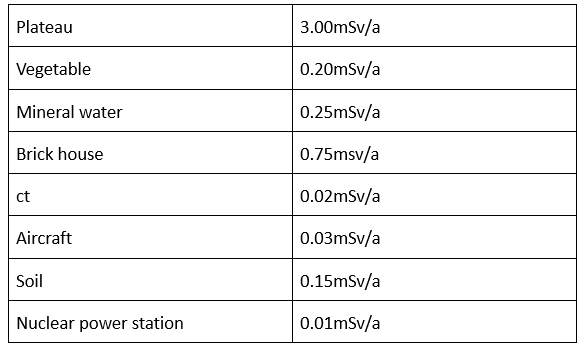
Ubiquitous radiation: Our foods, houses, sky and ground, mountains and vegetation, even bodies are radioactive. ≤250mSv/a, there is no harm to the human body.
Classification of Uranium Deposit
International Atomic Energy Agency (2013) divides the world uranium deposit into 7 industrial types. The estimated percentages of all types amounting to the world resources are as follows:
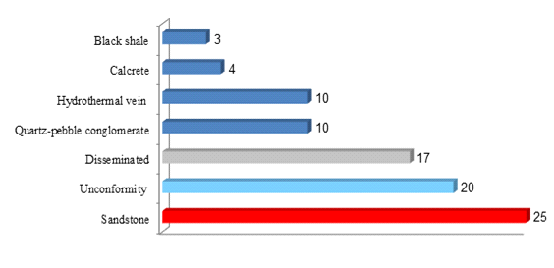
The disseminated uranium is mainly distributed both in Australia and Africa, the unconformity uranium ore mainly in Athabasca Basin and Canada, and the sandstone uranium deposit mainly in Central Asia.
Distribution of Uranium Resources in Major Countries
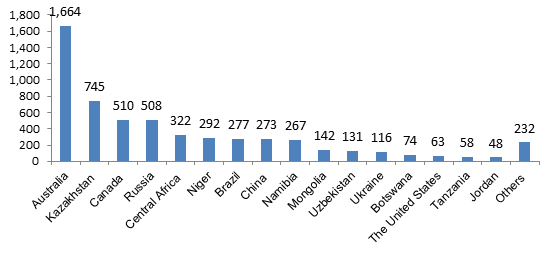
Source: OECD NEA & IAEA, Uranium 2016: Resources, Production and Demand ('Red Book').